Fusion Analytical Method Validation™
Analytical Method Validation Software
Automated Validation for LC
Works with:
- Waters® and Agilent LC Systems controlled by the Waters Empower™ 2 and 3 Chromatography Data Software (CDS)
- Agilent LC Systems controlled by the Agilent ChemStation and OpenLab CDS
- Thermo Scientific Dionex® Chromeleon® 6.8 and 7.2
All the experiments you need to support analytical method validation. Full LC experiment automation, automated calculation, graphing, and compliant reporting for:
Small Molecule — traditional pharmaceutical substances and products
Large Molecule — NEW — the only complete and automated method validation software for biopharmaceutical products (e.g. MAbs)
Non-LC Methods — used successfully for a wide variety of non-LC methods (e.g. QNMR, CE, GC-MS, LC-MS)
Fully aligned with the PhRMA Analytical Technical Group recommendation of a phased approach to analytical method validation in which early phase validation efforts are done upstream on a reduced set of validation elements appropriate to the stage of development:
Early Phase — for internal consumption to support and guide method development
Final Phase — submittal-quality: analysis and reporting meet all FDA/ICH guidance requirements

Key Benefits
- Full Automation – Phased Method Validation
- Early Phase – performance characterization supports development
- Final Phase – Aligned with FDA and ICH guidances
- 21 CFR 11 compliance support toolset
- Including E-records and E-signatures, full audit logging
- Workflow management system with E-review and E-approve loops
- Easy setup of experiments
- Create standardized workflow templates
- Facilitate rigorous practice and defensibility
- Simple documentation review and reporting
- Easy to defend and communicate
- Reports meet all FDA and ICH guidelines
Analysis and reporting meet all FDA/ICH Guidances
- Analytical Capability and System Suitability
[Characterizes the independent contributions of Sample Preparation and Sample Testing to Overall Precision] - Specificity
- Filter Validation
- Accuracy
- Linearity and Range / LOQ, LOD
- Repeatability* (intra-assay precision)
- Sample Solution Stability
[Stability for a given time period under prescribed conditions] - Accuracy / Linearity Repeatability / LOQ, LOD
[Can be run as a combined single experiment — ICH Q2(R2)] - Intermediate Precision and Reproducibility
[USP Ruggedness] - Robustness — done the right way!
Robustness Validation — DONE RIGHT!
You select the parameters to include in the automated robustness experiment. Fusion Method Validation (FMV) will automatically generate the robustness design, re-construct it in the CDS as ready-to-run methods and sequence, import the chromatogram results directly from the CDS, re-map them to the robustness study, and instantly analyze, graph, and report the results.
FMV robustness experiments let you use valid experiment ranges for accurate, defensible estimates of parameter effects. This avoids the risks associated with setting ranges equal to the expected variation ranges of your instrument parameters.
FMV robustness analysis wizard lets you set:
- expected parameter variation ranges
- acceptable performance limits for each key response
The wizard then accurately determines and reports the method’s true robustness for
- Statistical Significance
- Practical Significance
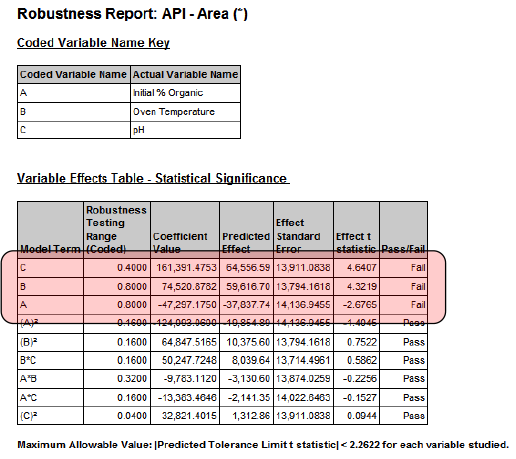
Robustness Validation — Statistical Significance Testing — Model Coefficients
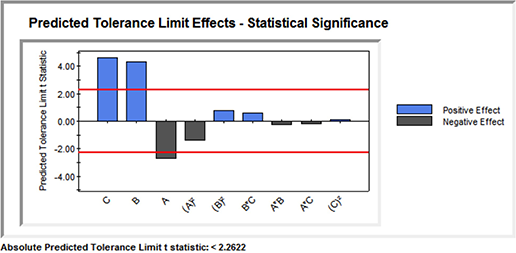
Robustness Validation — Practical Significance Testing — Effects Magnitude

Robustness Validation — Visualization of Robust Design Space (MODR) and PARs

Pharma Customer Benchmarking
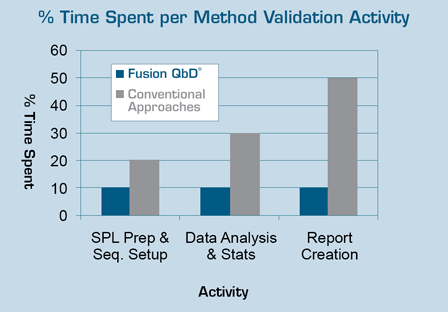
To benchmark time savings using FMV versus current practice, a senior analytical chemist at an international pharmaceutical account used FMV to complete a series of Early Phase and Final Phase method validation experiments. The entire method validation exercise took less than 12 hours using Fusion QbD.
Work records showed that on average — from SOP planning and experiment design construction to final reporting — using "current practice" this project would have required more than two weeks of analyst time.